Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Android | Pandora | iHeartRadio | TuneIn | RSS
With Dr. Prem Kandiah, neurointensivist, transplant intensivist, and ammonia enthusiast, we explore the physiology of hyperammonemia, the nuances of its measurement and interpretation, and unpack some less-recognized causes, including infection by urease-producing organisms and malnutrition/gastric bypass.
Learn more at the Intensive Care Academy!
References and resources
- Kamel, A.Y., Shah, P., Pipek, L.Z. et al. Nutritional Emergencies. Curr Surg Rep 13, 30 (2025). https://doi.org/10.1007/s40137-025-00465-9:
- Hyperammonemia cheatsheet from Prem:
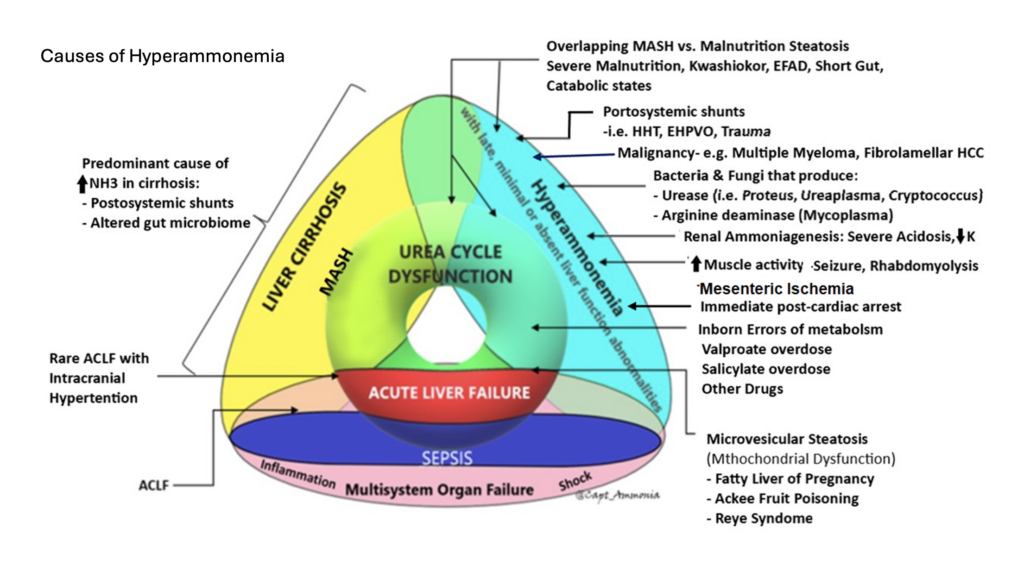
Acute Liver Failure
Overall management of ALF
- Bernal, W. and J. Wendon, Acute liver failure. N Engl J Med, 2014. 370(12): p. 1170-1.
- Bernal, W., et al., Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol, 2013. 59(1): p. 74-80.
- Kandiah PA, Nanchal R, Subramanian RM. Acute Liver Failure. In: Schmidt GA, Kress JP, Douglas IS. eds. Hall, Schmidt and Wood’s Principles of Critical Care, 5th Edition. McGraw Hill; 2023. Accessed August 09, 2023.
CRRT in ALF
- Warrillow S, Fisher C, Tibballs H, et al. Continuous renal replacement therapy and its impact on hyperammonaemia in acute liver failure. Crit Care Resusc 2020;22:158-165.
- Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ, Group USALFS. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology 2018;67:711-720.
Osmolar Shifts in ALF
- Liotta EM, Romanova AL, Lizza BD, Rasmussen-Torvik LJ, Kim M, Francis B, et al.
- Osmotic shifts, cerebral edema, and neurologic deterioration in severe hepatic encephalopathy. Crit Care Med. (2018) 46(2):280–9. doi: 10.1097/CCM.0000000000002831
Ammonia toxicity in Liver Failure
- Dasarathy S, Mookerjee RP, Rackayova V, Rangroo Thrane V, Vairappan B, Ott P, et al. Ammonia toxicity: from head to toe? Metab Brain Dis. (2017) 32(2):529–38. doi: 10.1007/s11011-016-9938-3
- Guo, R.M., et al., Brain MRI findings in acute hepatic encephalopathy in liver transplant recipients. Acta Neurol Belg, 2018. 118(2): p. 251-258
- Kumar G, Taneja A, Kandiah PA. Brain and the Liver: Cerebral Edema, Hepatic Encephalopathy and Beyond. Hepatic Critical Care. 2017 Aug 7:83–103. doi: 10.1007/978-3-319-66432-3_8. PMCID: PMC7122599.
Ammonia in Cirrhosis
- Gallego JJ, Ammonia and beyond – biomarkers of hepatic encephalopathy. Metab Brain Dis. 2025 Jan 15;40(1):100. doi: 10.1007/s11011-024-01512-7. PMID: 39812958; PMCID: PMC11735499.
Ammonia in in post bariatric surgery hyperammonemia
- Kamel AY, Shah P, Pipek LZ, Shah A, Kandiah PA. Nutritional Emergencies. Current Surgery Reports. 2025;13(1):30. DOI10.1007/s40137-025-00465-9
Post Lung Transplant Hyperammonemia
- Kamel, A.Y., et al., Hyperammonemia After Lung Transplantation: Systematic Review and a Mini Case Series. Transpl Int, 2022. 35: p. 10433.
Post cardiac arrest Hyperammonemia
- Nojima T et al. Can Blood Ammonia Level, Prehospital Time, and Return of Spontaneous Circulation Predict Neurological Outcomes of Out-of-Hospital Cardiac Arrest Patients? A Nationwide, Retrospective Cohort Study. J Clin Med. 2022 May 4;11(9):2566. doi: 10.3390/jcm11092566. PMID: 35566692; PMCID: PMC9105173.
Ammonia Bowel Ischemia
- Watari M et al. Ammonia determination as an early indicator in experimental superior mesenteric artery occlusion. Hiroshima J Med Sci. 1997 Dec;46(4):159-67. PMID: 9538566.
Study on undifferentiated causes of hyperammonemia
- Maquet J et al. Clinical, biochemical, and molecular findings in adults with hyperammonemia: A French bi-centric retrospective study. Mol Genet Metab. 2025 Sep-Oct;146(1-2):109223. doi: 10.1016/j.ymgme.2025.109223. Epub 2025 Aug 13. PMID: 40834544.
- Sakusic A, Features of Adult Hyperammonemia Not Due to Liver Failure in the ICU. Crit Care Med. 2018 Sep;46(9):e897-e903. doi: 10.1097/CCM.0000000000003278. PMID: 29985210; PMCID: PMC6095817.
Takeaway lessons
- Effective ammonia clearance requires liver function, kidney function, gut function, and presence of skeletal muscles. It can also be compromised by A-V shunting bypassing the portal venous system (i.e. from enteric to systemic circulation; ask your radiologist to look for this as they may not otherwise report it).
- Measuring the plasma ammonia level is not too technically difficult, but does have a time limit; allowing a sample to sit may falsely elevate the result. It’s therefore tough to do for outpatients. For inpatients, however, unless it gets forgotten on a table, it is generally not so difficult. Arterial vs venous doesn’t matter.
- The peak ammonia on admission for cirrhotics is not as relevant as their cumulative ammonia burden over time (though of course this is not measurable). While difficult to interpret for those patients, a very high number is still very toxic, a very normal level probably absolves hepatic encephalopathy as a cause of altered mental status, and trending a number in between may help confirm response to therapy (eg the number should come down). A persistently elevated ammonia despite adequate catharsis may indicate need for a more aggressive regimen, or other attention to the underlying cause; a common cause would be a patient with GI bleeding, where persistent blood in the gut can cause persistent ammonia elevation.
- Everyone agrees that ammonia should be followed in acute liver failure, where it correlates closely with risk of cerebral edema.
- Checking ammonia in the patient without clear liver failure, but unexplained altered mental status, is reasonable—though an abnormal result will require a thoughtful approach to explaining it.
- Cardiac arrest, especially with prolonged resuscitation, will reliably increase the ammonia level (if measured during or immediately after), as a direct effect of global ischemia.
- Gut ischemia can elevate ammonia. Enterocytes require glutamine for energy; by interrupting this pathway, they may generate ammonia via anaerobic metabolism, much as other cells might generate lactate.
- Medications, particularly valproate, can elevate ammonia.
- Any elevated muscle activity or muscle breakdown, such as seizure or rhabdomyolysis, can generate nitrogen and hence transient hyperammonemia.
- Any sarcopenia predisposes to hyperammonemia, as skeletal muscle is too sparse to reliably clear ammonia.
- Inborn errors of metabolism involving the urease cycle are uncommon in adults but something to consider, especially if genetic testing is available to you.
- Severe malnutrition, most often due to gastric bypass, can cause hyperammonemia. This is probably due to multifactorial causes, including sarcopenia and muscle catabolism, but malnutrition seems to induce a true hepatic steatosis is well (reversible if nutrition is restored). Radiographically and clinically this looks like MAFLD/MASH, but is not caused by metabolic syndrome or obesity, but the opposite state of malnutrition. (This phenomenon is also seen in Kwashiorkor.) Probably this is due to some synthetic dysfunction affecting beta oxidation and lipid metabolism, and/or absence of essential fatty acids—not clear.
- Urease-producing bacteria can generate ammonia. This will usually be transient, since antibiotics will readily kill them. However, with a deep-seated infection such as an abscess, this may not be true and should be considered as an ongoing ammonia source. Examples include: Staph epidermidis and Staph saprophyticus, Helicobacter Pylori, Klebsiella, Nocardia, Cryptococcus, Pseudomonas spp., Corynebacterium, Proteus penneri, Providencia stuartii, and Morganella morganii
- An especially important urease producer is ureaplasma, an atypical organism similar to mycoplasma, usually causing UTI or even STI. This will often not cause other signs of clinical infection, but can be a cause of ammonia production, and will not be grown on routine cultures (PCRs are needed on urine or BAL, or the Mayo Clinic has a blood PCR—all send-outs), nor covered with routine antimicrobials (atypical coverage, such as doxycycline, azithromycin, or levofloxacin is needed). This infection mostly occurs in the immunosuppressed, such as transplant patients, where it can cause occult pneumonia (an important and morbid cause of post-transplant hyperammonemia); consider it as well in anybody on rituximab or similar immunosuppression. It can also cause “sterile” joint effusions, so a patient on rituximab whose joint is tapped and grows inflammatory-but-aseptic fluid may be presumed to have progression of an underlying rheumatologic disease, have their immunosuppression escalated, and then end up with disseminated ureaplasma.
- For the hyperammonemic due to malnutrition/gastric bypass: nourish with glucose and fats while limiting or holding protein completely in the acute period. This can be parenteral initially, as it’s easy to titrate protein in that fashion. Check and/or supplement everyone with micronutrients likely to be insufficient: high-dose thiamine, B6, L-carnitine, copper, zinc. Once ammonia stabilizes and clears, start to introduce protein cautiously, with the goal of eventually restoring an anabolic state to reverse the underlying steatosis. Go slow weaning everything to avoid rebounds.
- Refractory hyperammonemia can be treated with dialysis (CRRT), though data is limited in this setting. Starting earlier may make sense, as osmolar clearance may be rapid once initiated and could precipitate cerebral edema if the osm load is already high. Some would add hypertonic saline with CRRT to try and mitigate this rapid osm drop, a common tactic in the ALF realm.
- A common cause of proximal decompensation for these people is when micronutrient deficiencies starts to reduce gut motility, leading to poor tolerance of oral nutrition and anorexia. Once you fix these and the gut allows oral intake, their nutritional status can improve.
- Lactulose and rifaximin can have some temporizing role in these patients, but a very short-acting one. They may also tend to worsen nutrition by accelerating gut transit time and filling the small-volume stomach.
- The urease cycle scavengers sodium phenylacetate-sodium benzoate may help accelerate clearance of ammonia, if renal function is intact (they convert ammonia to PAGN which can be renally cleared). However, they are not a magic solution, take time to work, and do not address the underlying problem.
