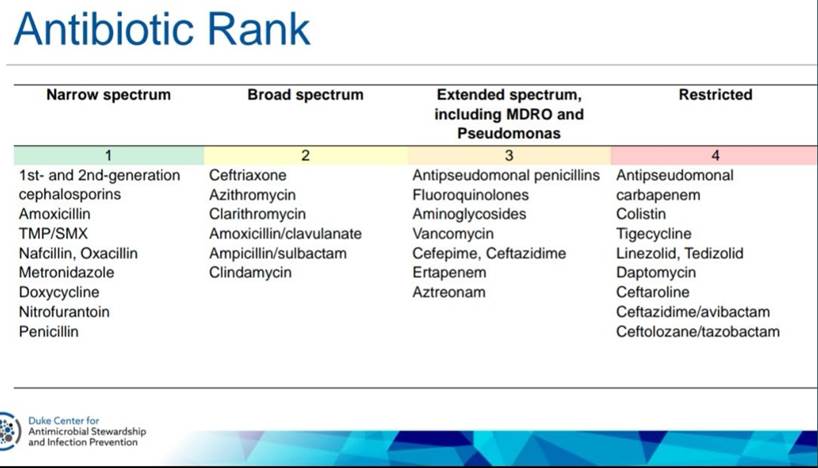
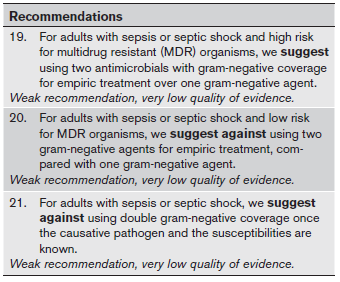
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Android | Pandora | iHeartRadio | TuneIn |
The art of taking a critically ill, heavily sedated, floridly delirious patient on aggressive vent support and pulling them out of the loop of sedation, immobility, and delirium. With Kali Dayton, ACNP-BC (@HomeIcu), critical care nurse practitioner and host of the Walking Home from the ICU podcast, where she looks closely at these issues, including interviews with survivors describing their ICU experiences.
A spiritual successor to our talk with Dale Needham, this time focusing more on details and practical approaches.
Takeaway lessons
- Good care to optimize long-term function is also good care to optimize short-term survival and morbidity.
- Benzodiazepines are normally a poor choice for sedation given their deliriogenic properties. However, using benzos in patients with alcohol dependence is more appropriate. It can also be rationally used in more subacute patients in whom benzos aren’t being used as sedation but only as anxiolytics—i.e. low doses of an agent like clonazepam to preserve level of arousal but treat anxiety, much like it’s used in the outpatient setting.
- The combative behavior of delirious patients isn’t inexplicable; it’s a rational response to their perceived situation, which often involves vivid hallucinations of sexual abuse, torture, fractured realities, threats to loved ones, and similar horrors.
- Favor dexmedetomidine in patients who do need a sedative drip, but aim only for calm, not a depressed level of consciousness.
- Delirious (non-combative) patients can often still be mobilized to the extent tolerated, and it tends to actually improve their mental status. Limited activity is better than none. Concerns for self-extubation are usually easily managed by gentle restraint or redirection, as these patients are usually physically weak. A dexmedetomidine drip is not necessarily a contraindication to mobility.
- Ventilator settings are rarely a contraindication to mobility. Increased FiO2 may be necessary and is acceptable. Modes can be adjusted as needed. While exertion may increase respiratory needs, this change is rarely precipitous or “dangerous”; adjustments can simply be made as needed.
- Fatigue induced by exercise is a good thing and may facilitate further reduction in sedation. Allow patients to nap, but not too long (to preserve a normal sleep-wake cycle). In an ideal world, aim for three mobility sessions daily: two on the day shift and one before bed.
- Proning does not necessarily mandate deep sedation and/or paralysis. It can be a “therapy, not a lifestyle,” with patients proned for a period of time (but awake and interactive) and then turned back up to perform mobility and other activities.
- Awake, non-delirious patients can require more “work” to mobilize etc, but in many other ways require less work. They understand their situation, can assist with their own care, protect their own tube, etc. They are part of their care, not working against it.
- With good care, tracheostomies are rarely needed for the most common reason of oversedation and weakness. Mobility and light sedation can be practiced without them. However, they may still occasionally be needed for truly refractory lung disease or anatomic issues like airway abnormalities.
- Sedated patients appear to be resting and comfortable, but they are not, and follow-up interviews reveal they are actually internally suffering from their delusions. On balance, most would much rather be awake but experiencing their true reality (even if bored or uncomfortable) rather than sedated and experiencing the horrific false realities of delirium.