Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Android | Pandora | iHeartRadio | TuneIn | RSS

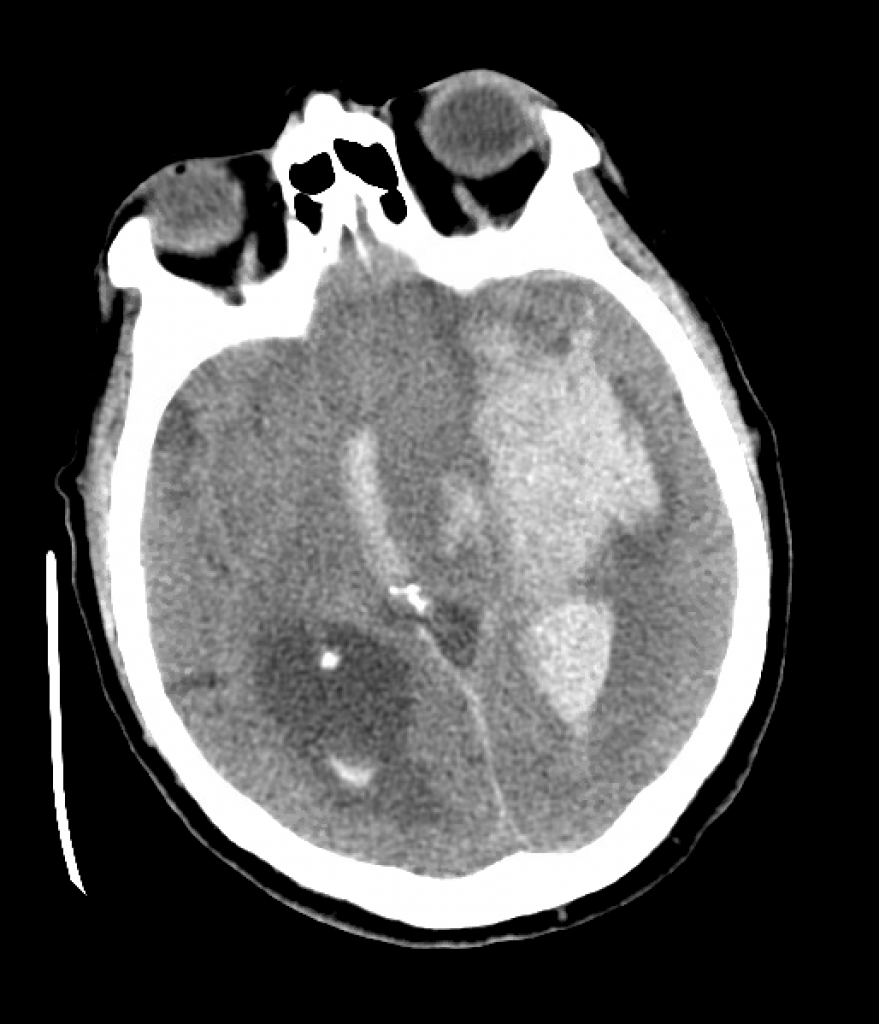
We review a case of massive intraparenchymal hemorrhage progressing to brain death, including the process of brain death testing and declaration, with Dr. Casey Albin (@CaseyAlbin), neurologist and neurointensivist, assistant professor of Neurology and Neurosurgery at Emory and part of the NeuroEmcrit team.
For 20% off the upcoming Resuscitative TEE courses (through July 23, 2022), listen to the show for a promo code for CCS listeners!
Takeaway lessons
- In general, in patients with good baseline function, it’s reasonable to be fairly aggressive with initial care, such as placement of intracranial pressure monitors, even if long-term goals of care are unclear—it can always be escalated.
- Although ICH score is associated with mortality, the original study allowed withdrawal of care at discretion of the clinicians, so the data may be tainted by self-fulfilling prophecy—withdrawal of care may lead to poor prognosis in some cases, not always the reverse.
- Sodium goals are ideally titrated to ICP (with invasive monitoring). In its absence it’s best to target clinical findings, unless you have tools like TCDs or optic nerve sheath ultrasound, or just frequent CT scans. Arbitrary sodium goals are rarely helpful.
- There is good evidence for decompressive hemicraniectomy for large MCA infarct IF the patient is young; it is less clear in the elderly. If it’s going to be done, do it early.
- If herniation is clear via ICP or imaging, don’t spare sedation for the sake of a neuro exam, unless you’re at the point of stepping back and assessing for long-term futility and possible brain death.
- 4-5 days into admission is often when families begin to understand the nature of a devastating neurologic injury. In some cases, discussion of futility and brain death may be initiated by families after doing their own research.
- The first step is holding sedation and waiting ~5 half-lives for confounding drugs to clear; impaired renal or hepatic clearance should be taken into account here. (Pharmacy may be helpful.) Paralysis should be held and train-of-four can be used to confirm. Drug levels can be used to confirm clearance of opioids, etc if needed.
- The law (Uniform Declaration of Death Act) doesn’t always agree with guidelines (while hospital policies may differ even further). The UDDA requires complete brain death, whereas the AAN’s guidelines don’t necessarily require pituitary death (patient need not be in DI), but all do require more than just brainstem death—for example, a locked-in patient would not qualify.
- Expect and manage DI, as hypovolemia and hypernatremia may make the patient too unstable to tolerate brain death testing. Consider a vasopressin drip, replace volume, etc.
- As the chest wall becomes denervated, it loses elastic recoil, while hypovolemia may cause very hyperdynamic cardiac function. The combination can cause strong chest wall vibrations which may autotrigger the ventilator, often confusing staff and family who believe the patient is breathing spontaneously.
- Perform brain death testing in a systematic, scrupulous manner. Print your hospital policy and use it as a formal checklist. You’ll need a bright penlight, a tongue depressor or Yankhauer catheter, a Q-tip or gaue for corneal reflexes, 50 ml x2 of ice-cold water and a syringe with an IV catheter on the tip for cold calorics, and some kind of insufflation catheter or a T-piece for apnea testing.
- Pitfalls: remember to test corneals by touching the actual cornea, not the sclera. Cold calorics are performed by irrigating the ear canal and watching for gaze deviation (any deviation shows brainstem activity). Gag reflex must be checked all the way in the back of the oropharynx with vigorous stimulation. Cough and pain responses must also be checked with substantial stimulation. Warn family ahead of time about the possibility of purely reflexive triple flexion.
- Consider bringing the family to watch, which helps encourage transparency. Warn them ahead of time that if the test is confirmatory, it will indicate the patient is dead by brain criteria.
- You generally want an arterial line for the apnea test, and have vasopressors running and ready to maintain the SBP >100. Put the patient on 100% FiO2 and get a baseline ABG showing normocapnia and a PAO2 >200. (If the patient has a baseline elevated PACO2, follow your local policy.) Oxygenate the patient passively, such as by inserting an insufflation catheter hooked up to oxygen down the ET tube after disconnecting the ventilator. Uncover the patient’s chest and watch for chest rise.
- A confirmatory apnea test is one where the PACO2 rises by 20 points, without any clinical signs of breathing; hence the team needs to be in the room, physically observing the patient. An equivocal test is one where the test cannot be completed or the PCO2 fails to adequately rise to confirm adequate levels. Most tests are completed by 10 minutes, but start sending blood gasses earlier than that (e.g. at 6, 8, 10 minutes), as you may need to terminate the test due to instability while waiting for the most recent gas and you’ll want to know if the patient had finished.
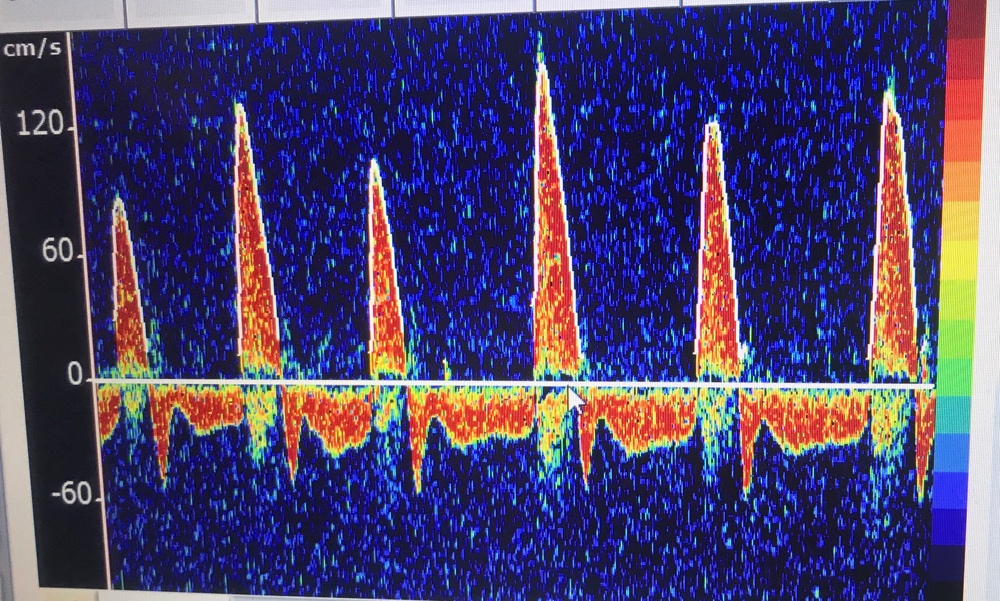
- Confirmatory/ancillary tests can be done if the clinical and apnea tests cannot be done, or are not completely definitive due to confounding factors. They can include TCDs, nuclear flow studies, or EEG if specialized equipment and readers are available. Catheter-directed 4-vessel cerebral angiography is another option, but CTA/MRA are not. Most of these tests are looking for intracranial circulatory arrest, i.e. lack of blood flow to the brain—dead cells have no metabolic demand and shunt blood away.
- Perform brain death testing as soon as clinically appropriate; they only become more unstable.
Resources