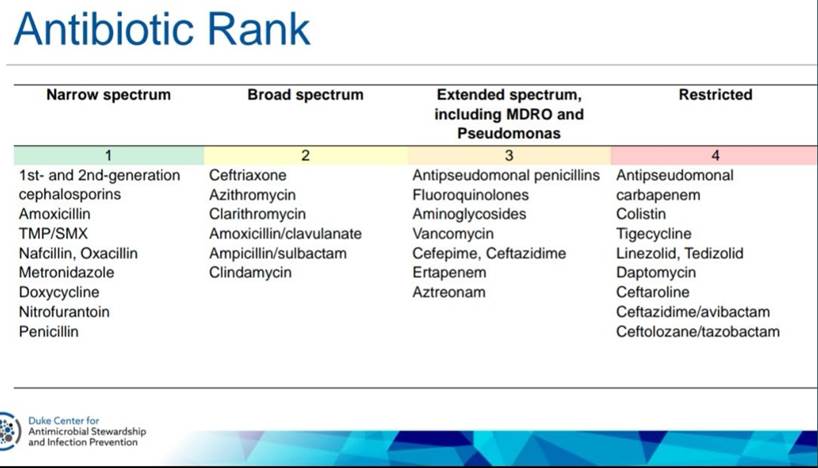
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Android | Pandora | iHeartRadio | TuneIn |
An overview of VV ECMO with a focus on COVID-19, with Dr. Kimberly A. Boswell (EM and CCM) of the University of Maryland, perhaps the busiest center in the country for COVID-related ECMO. We discuss evaluating for candidacy, induction, maintenance, weaning, and general approaches to the COVID patient.
Takeaway lessons
- The limited amount of ECMO resources has led to narrowing of criteria. Maryland has reduced their standard upper age limit from <65 to <55, BMI of <40, mechanical ventilation duration <7 days (formerly <10). Also consider other organ failures, as well as duration of symptoms—not just intubation—as a prolonged pre-intubation course suggests a late, potentially fibroproliferative phase of disease which may not be responsive to ECMO.
- Almost all COVID cases at Maryland have been VV ECMO; they have very rarely considered VA ECMO. The most obvious indication for the latter would be right heart failure, but in most cases, they would be more likely to use VV ECMO (or other medical therapies, such as inhaled vasodilators or diuresis) to unload the right heart, or else to consider severe cardiogenic shock to actually be a contraindication to ECMO (as it suggests a late stage of disease less likely to respond to aggressive care).
- There is no obvious timeframe which is “too early,” but patients already
at ECMO-ready centers might reasonably wait longer to go on bypass, as it can be done quickly and safely when necessary without requiring interfacility transport. - Cannulation can be done by whomever is skilled and trained, such as cardiac surgery, trauma surgery, trained intensivists, etc.
- For VV ECMO, Maryland likes to cannulate the right IJ and right femoral veins, or perhaps the left femoral if needed. They prefer not to cannulate bilateral femorals, and prefers not to use dual-lumen IJ catheters (the Avalon bi-caval catheter), as flow is often not adequate.
- Anticoagulate most patients on VV ECMO with heparin to a PTT of 45-55. VA ECMO can go to 60-80. ECMO without anticoagulation can be done if there are bleeding issues, however.
- Maryland generally does not titrate FiO2 on the sweep gas. After induction, titrate the sweep; the goal is usually to correct hypercarbia over 6–8 hours, not all at once.
- Flow rates at least 4 L/min, unless more is required for hypoxia. RPM <4000 is usually the starting goal.
- Prone even while on pump for lung-protective reasons. Chest PT is good too. Prone first for 6-8 hours to ensure tolerance and skin integrity, then do around 4 more sessions of 16 hours each, as a starting goal.
- Ventilator settings on VV ECMO can be walked back after induction. Historically they used PEEP 10, PIP 10, RR 10. In heavily consolidated COVID patients, some need more pressure to maintain some degree of recruitment, such as PEEP 15 and PIP 10.
- Inhaled vasodilators can be continued or weaned depending on right heart function. Diurese until you develop flow problems (suction events) on the pump, a useful indicator of low intravascular volume.
- Have a low threshold to deeply sedate and/or paralyze while on pump to optimize synchrony and facilitate proning. However, Maryland likes to perform “partial paralysis,” with just enough NMB to achieve goals; respiratory rates below 20 or so are considered acceptable.
- Early tracheostomy is reasonable, but persistently high requirements for ventilator pressures often pushes it back.
- Hypoxemia can occur in VV ECMO patients due to too much flow through the native circulation and shunted lungs. In such cases, beta blockade may actually improve systemic oxygenation.
- Plasma free hemoglobin levels may be a useful marker that changing your oxygenator could improve gas exchange.
- Decannulate at the bedside when ready, watch them for 24 hours, then boot them out of the ICU; they’re ready.
- 65%+ of COVID ECMO patients at Maryland are surviving. Data remains slim, but there seems to be decent results in a well-selected population.
- In rare cases, patients who neither die nor recover may become candidates for lung transplant.